Groundbreaking non-pharmaceutical and clinically validated therapy for fibromyalgia.
Remedee Labs aims to make its solution a standard treatment for fibromyalgia
About the FIBREPIK study
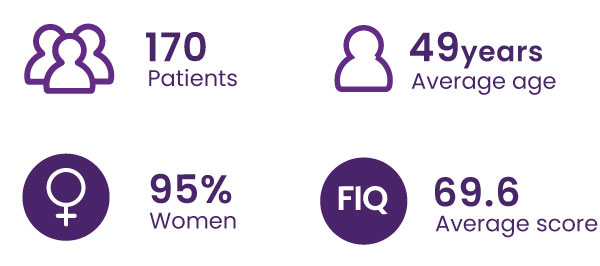

FIQ Score ≥39/100, from 8 French chronic pain care centers
The FIQ questionnaire is the standard clinical test used to assess the impact of fibromyalgia on patients’ quality of life. It covers physical activity, work, depression, anxiety, sleep, pain, stiffness, fatigue, and feelings of well-being.
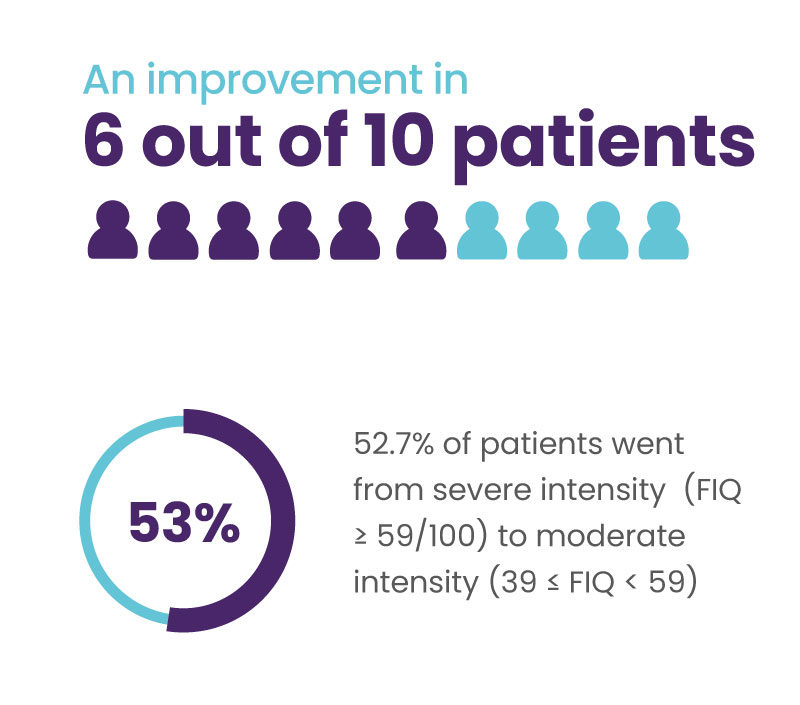
A 14% decrease in the total FIQ score is considered clinically significant (Bennett et al. 2009.
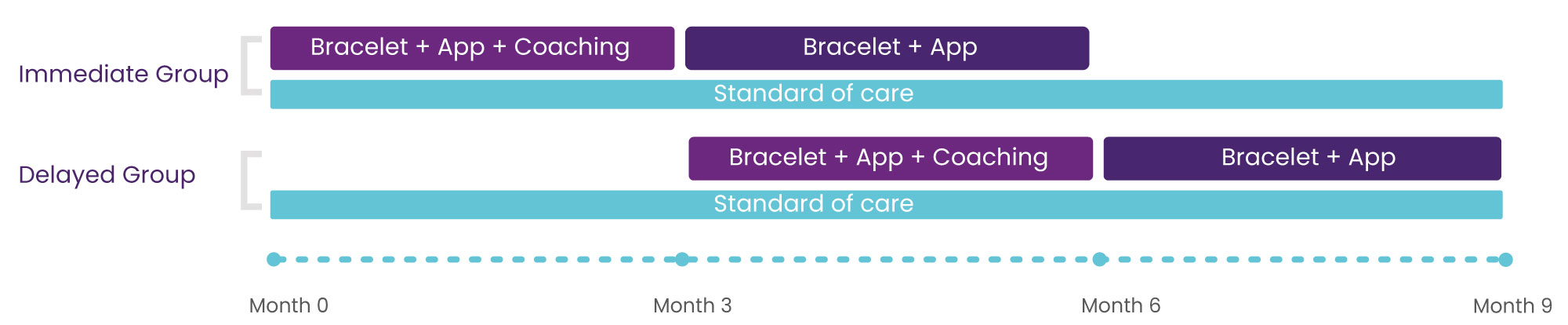
Procedure: 2 Groups
The Remedee Labs Therapy
1st millimeter wave endorphin stimulator
- Simple · Non-invasive · Painless
- 30-minute sessions
- 3 times per day
Coaching
- 4 meetings (D0 · D7 · D30 · D60
- Training + Support
- Progress monitoring
Mobile Application
- Session tracking
- Step tracking
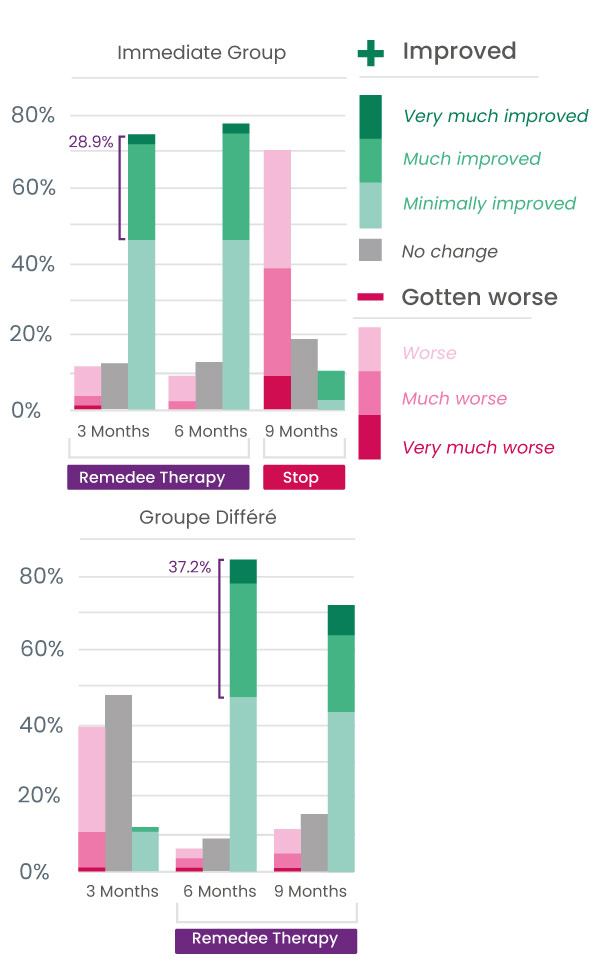
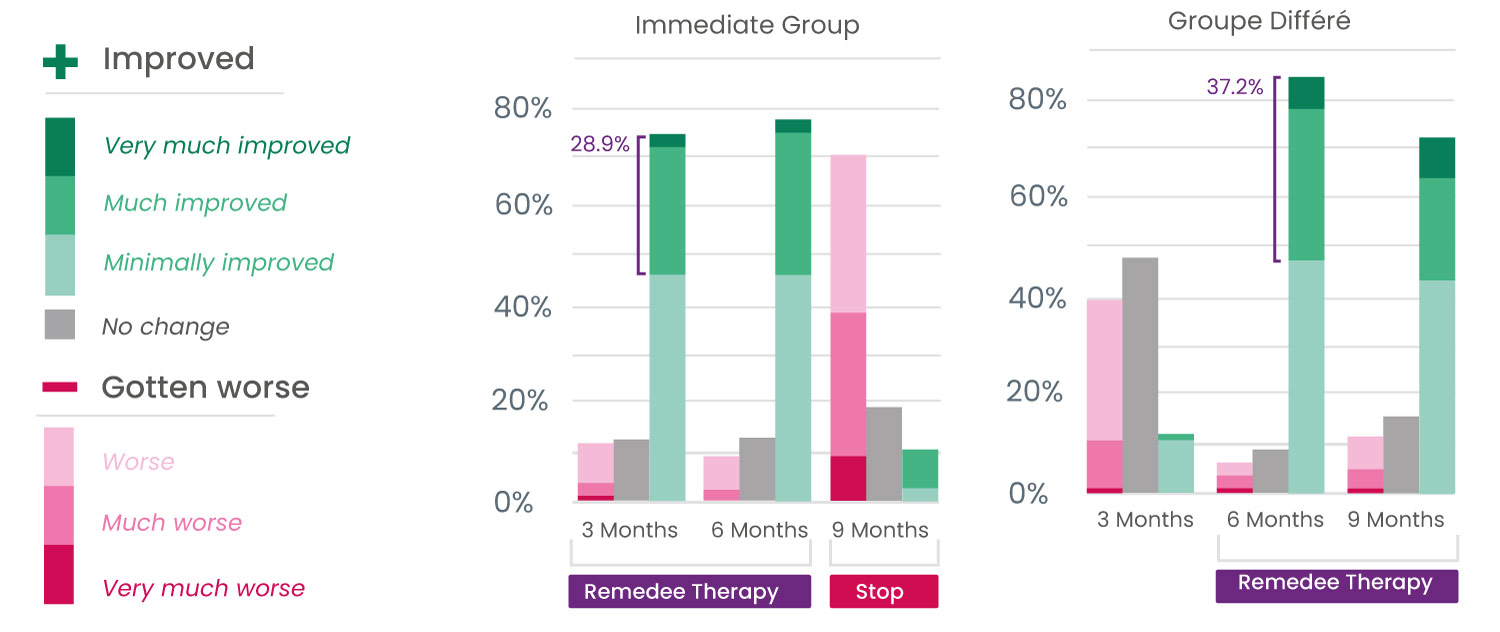
Study Results
Significant improvement in quality of life for 6 out of 10 patients
Improvements in the main symptoms of fibromyalgia
Participants also showed a significant improvement in the main symptoms of fibromyalgia, with a significant decrease in pain, fatigue, anxiety, and depression, as well as improved sleep quality.

Sleep & Fatigue

Pain

Depression & Anxiety
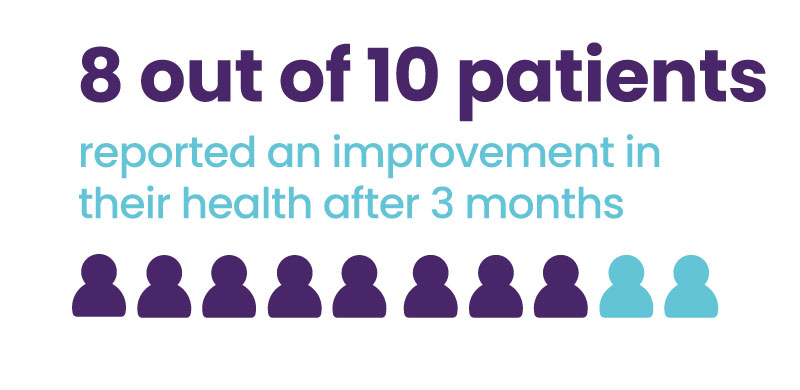
Patients confirmed the improvement
Over 75% of patients reported an improvement in their health (Patient impression of change scores) after 3 months of use.
Perceived benefit of therapy was measured using the Patient Global Impression of Change (PGIC) questionnaire.

Providing care for patients suffering from fibromyalgia is currently a major public health challenge. The positive results of the FIBREPIK study are very encouraging. Remedee Labs’ solution fits perfectly into a coordinated care approach, in which non-pharmaceutical treatments are the first choice
Docteur Caroline Maindet
Pain Specialist at Grenoble Alpes University Hospital and lead investigator for the FIBREPIK study
Fibromyalgia: an invisible and severely debilitating disease
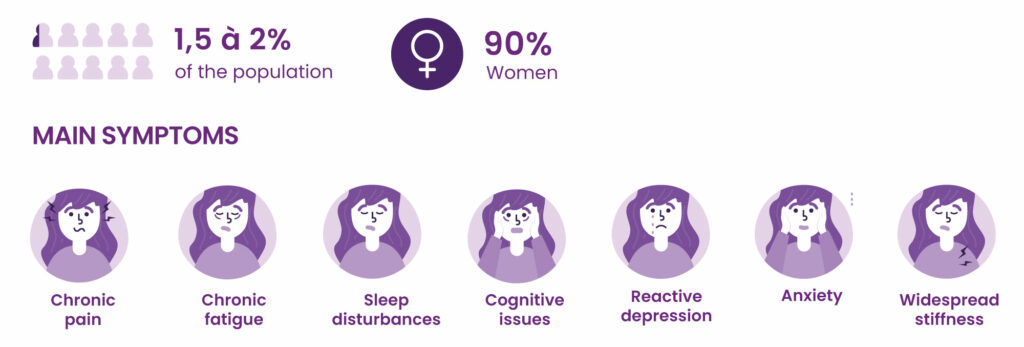
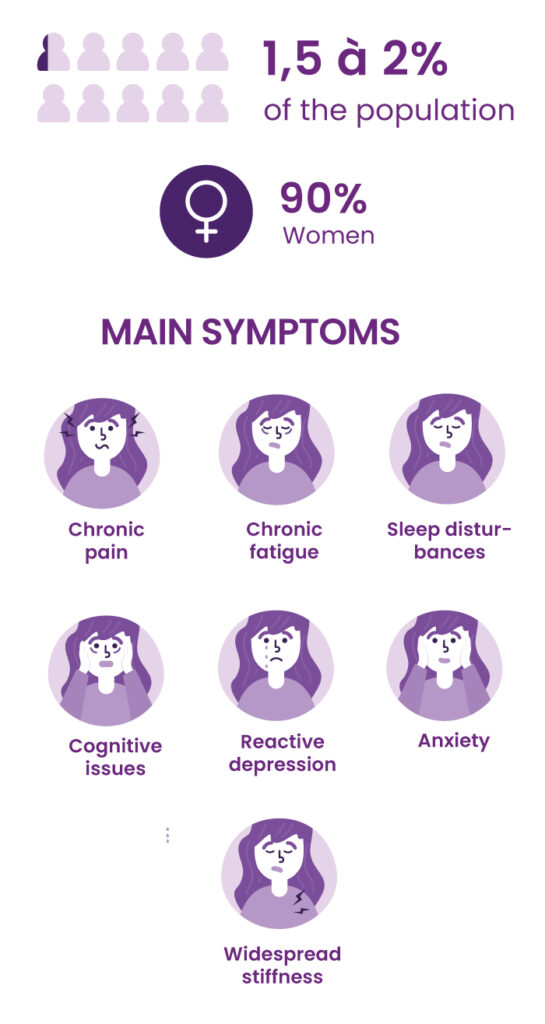
Fibromyalgia is a form of chronic widespread pain, associated with hypersensitivity to pain and a range of other symptoms, particularly sleep and mood disorders. It has a severe impact on quality of life and social and professional activities.
According to the collective expertise of INSERM, the French national medical research institute, about 2% of the French population has fibromyalgia. Women make up the majority of patients (80% to 90%). There is currently no specific treatment for fibromyalgia.
Management of the condition is based on multidisciplinary expertise. The standard of care for fibromyalgia essentially consists of non-pharmaceutical treatments, particularly adapted physical activity (APA) led by a professional. Counseling is also an important component of care. It is not simply a default option; in fact, it aims to improve pain modulation, which is altered by fibromyalgia. In most cases, it involves cognitive behavioral therapy (CBT) and patient education. Pharmaceutical treatments such as pain relievers, anti-depressants and anti-seizure drugs can only be prescribed at a later stage; however, their use must remain occasional.
« Today, there is no dedicated treatment for fibromyalgia patients. They need multi-disciplinary care centered on non-pharmaceutical treatment. Unfortunately, that type of care is not always available at chronic pain research and treatment centers due to the lack of dedicated resources. That means the non-pharmaceutical therapy offered by Remedee Labs, which has shown very positive clinical results, is a major advance in fibromyalgia management because it provides a holistic approach and creates a personal connection through personalized coaching. Since fibromyalgia patients often struggle financially, we hope that this therapy will be covered by social security very soon”
Nadine RANDON



